Loading...
Worried about iNO safety in your NICU?
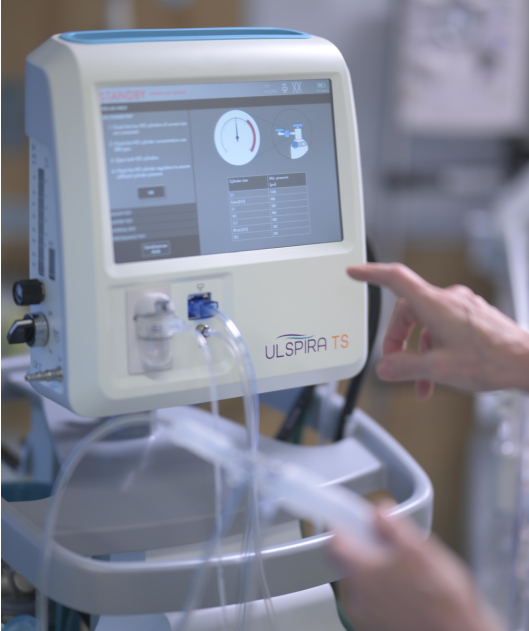
Now you can all breathe easier — the ULSPIRA TS™ is specifically designed to simplify iNO treatment and increase patient safety
An integral part of the ULSPIRA Solution™ is the ULSPIRA TS, our nitric oxide therapy system designed for ease of use and patient safety in mind, providing simplified treatment management to bring inhaled NO therapy to the next level. Our therapy system is supported by our team of clinical applications specialists who are trained RTs with years of nitric oxide therapy experience.

Now you can take advantage of special pricing and terms pre-negotiated by Premier for all your iNO therapy needs. Complete the form for more information.
With the ULSPIRA TS, you can:
Save time and start therapy fast
Simplify treatment management
Focus on patient safety
Depend on clinical support for your team
24/7/365 access to Clinical Application Specialists (RRT) via dedicated hotline: 833-ULSPIRA (833-857-7472)
Pair the ULSPIRA TS with ULSPIRA™ nitric oxide gas
ULSPIRA (nitric oxide) gas for inhalation is a vasodilator drug indicated to improve oxygenation and reduce the need for extracorporeal membrane oxygenation. It's prescribed for use in term and near-term (>34 weeks gestation) neonates with hypoxic respiratory failure associated with clinical or echocardiographic evidence of pulmonary hypertension in conjunction with ventilatory support and other appropriate agents. ULSPIRA is available in an 800 ppm concentration.

ULSPIRA (nitric oxide) gas, for inhalation, is a vasodilator indicated to improve oxygenation and reduce the need for extracorporeal membrane oxygenation in term and near-term (>34 weeks gestation) neonates with hypoxic respiratory failure associated with clinical or echocardiographic evidence of pulmonary hypertension in conjunction with ventilatory support and other appropriate agents.
Contraindications
ULSPIRA is contraindicated in neonates dependent on right-to-left shunting of blood.
Warnings and Precautions
Adverse Reactions
The most common adverse reaction of ULSPIRA is hypotension.
Drug Interactions
Nitric Oxide donor compounds may increase the risk of developing methemoglobinemia.
Use With Delivery System
ULSPIRA must be administered using a calibrated FDA-cleared Nitric Oxide Delivery System operated by trained personnel. Only validated ventilator systems should be used in conjunction with ULSPIRA.
Adverse Events
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Please see full ULSPIRA prescribing information for additional ULSPIRA safety and risk information.
Intended Use
The ULSPIRA TS Nitric Oxide Therapy System is intended for use by healthcare professionals for the delivery of nitric oxide (NO) and the monitoring of inspired NO, NO2 and O2 concentrations for a patient undergoing inhaled Nitric Oxide (iNO) therapy.
ULSPIRA TS must only be used in accordance with the indications, contraindications, warnings and precautions described in the nitric oxide drug packaging inserts and labeling, and is indicated for use in term and near-term (>34 weeks gestation) neonates with hypoxic respiratory failure associated with clinical or echocardiographic evidence of pulmonary hypertension. The primary targeted clinical setting is the Neonatal Intensive Care Unit (NICU) and secondary targeted clinical setting is the transport of neonates. Refer to this material prior to use.
In the case of an abrupt stop of inhaled NO gas delivery, withdrawal (rebound pulmonary hypertension) may occur. Possible side-effects of the inhaled NO gas therapy are further described in the nitric oxide drug packaging inserts and labeling.
ULSPIRA TS can be used with respiratory devices. Make sure that ULSPIRA TS is validated with the respiratory device you use. Validated respiratory devices are listed in the User's Manual.
Device Warnings:
Rx Only
ULSPIRA Indication and Important Safety Information
ULSPIRA (nitric oxide) gas, for inhalation, is a vasodilator indicated to improve oxygenation and reduce the need for extracorporeal membrane oxygenation in term and near-term (>34 weeks gestation) neonates with hypoxic respiratory failure associated with clinical or echocardiographic evidence of pulmonary hypertension in conjunction with ventilatory support and other appropriate agents.
Contraindications
ULSPIRA is contraindicated in neonates dependent on right-to-left shunting of blood.
Warnings and Precautions
Adverse Reactions
The most common adverse reaction of ULSPIRA is hypotension.
Drug Interactions
Nitric Oxide donor compounds may increase the risk of developing methemoglobinemia.
Use With Delivery System
ULSPIRA must be administered using a calibrated FDA-cleared Nitric Oxide Delivery System operated by trained personnel. Only validated ventilator systems should be used in conjunction with ULSPIRA.
Adverse Events
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Please see full ULSPIRA prescribing information for additional ULSPIRA safety and risk information.
Please complete the form to connect with an expert at Airgas.
Talk to an expert about your gases, hardgoods and safety needs.